Give IUPAC names for the following aldehydes and ketones:
b.

 Verified step by step guidance
Verified step by step guidance


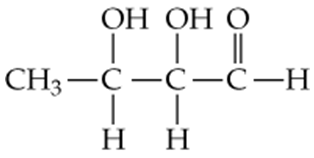
Give IUPAC names for the following aldehydes and ketones:
b.
The following names are incorrect. What is wrong with each?
a. 1-Pentanone
The following names are incorrect. What is wrong with each?
b. 2-Butanal
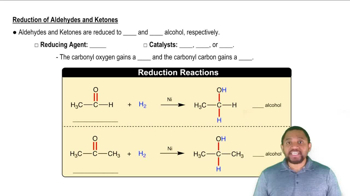
Draw the structures of the products formed when the following compounds react with a reducing agent.
a.
Write the structures of the hemiacetal or hemiketal that result from reactions (a) and (b). Write the structures of the complete hydrolysis products of the acetal or ketal in (c) and (d).
a. Acetone + Ethanol → ?
Cyclic hemiacetals commonly form if a molecule has both an alcohol group and a carbonyl group elsewhere in the same molecule, especially if they are four or five carbons apart. What is the structure of the hydroxy aldehyde from which this hemiacetal might form?