Which of the following compounds will react with Tollens' reagent? With Benedict's reagent?
a. Cyclopentanon
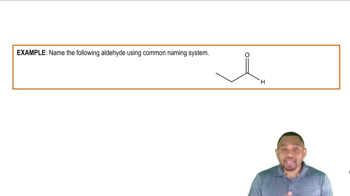
b. Hexanal
c.

 Verified step by step guidance
Verified step by step guidance
Which of the following compounds will react with Tollens' reagent? With Benedict's reagent?
a. Cyclopentanon
b. Hexanal
c.
Draw the structures of the products formed when the following compounds react with a reducing agent.
a.
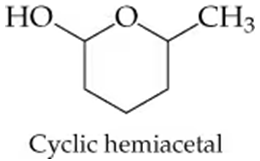
Write the structures of the hemiacetal or hemiketal that result from reactions (a) and (b). Write the structures of the complete hydrolysis products of the acetal or ketal in (c) and (d).
a. Acetone + Ethanol → ?
What two products result from the complete hydrolysis of this cyclic acetal?
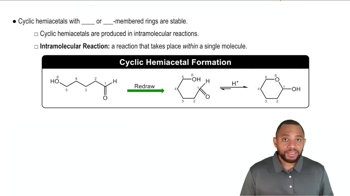
Acetals and ketals are usually made by reaction of an aldehyde or ketone with two molecules of a monoalcohol. If an aldehyde or ketone reacts with one molecule of a dialcohol, however, a cyclic acetal or ketal results.
b. Draw the cyclic ketal formed when the hemiketal from part (a) reacts with the ―OH labeled in blue.
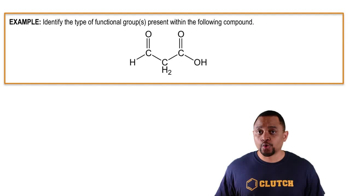
Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone.