Textbook Question
Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(b)
560
views

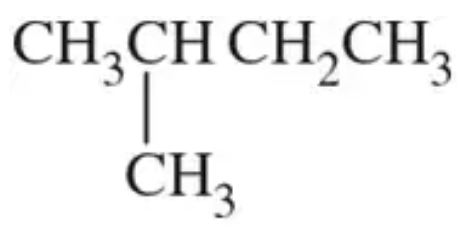
 Verified step by step guidance
Verified step by step guidance


Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(b)
Mark the chiral centers in the following molecules, if any, with an asterisk (*):
(d)
Convert each of the Lewis structures shown into a condensed structural formula:
(c)
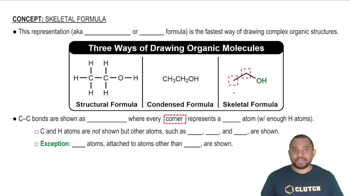
Convert the condensed structures shown to skeletal structures.
(c)
Convert the condensed structures shown to skeletal structures.
(b) CH3CH2CH2CH2OH
Convert the skeletal structures shown to condensed structures.
(a)