Identify the monosaccharide that fits each of the following descriptions:
(a) in combination with glucose produces the disaccharide lactose

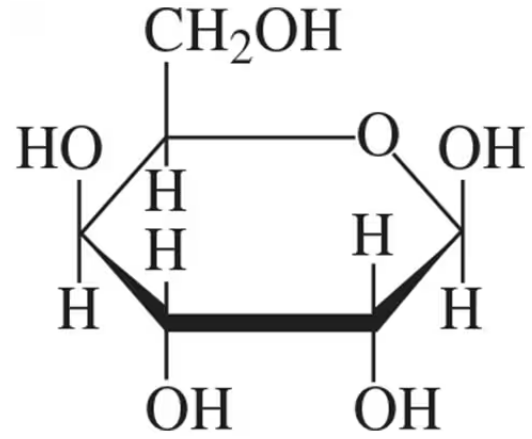
 Verified step by step guidance
Verified step by step guidance



Identify the monosaccharide that fits each of the following descriptions:
(a) in combination with glucose produces the disaccharide lactose
Indicate whether the following statements apply to type 1 or type 2 diabetes:
(a) most cases begin in youth
Indicate whether the following statements apply to type 1 or type 2 diabetes:
(c) can be managed with diet and exercise
Draw the ⍺ and β anomer of D-talose in pyranose ring form:
The sugar alcohol ribitol is a component of the vitamin riboflavin and the energy transfer molecule FAD. Ribitol is formed when the monosaccharide ribose undergoes reduction at carbon 1. Draw the structure of ribitol.
The sugar alcohol erythritol is often included in low-calorie sweeteners. It is 70% as sweet as table sugar. Erythritol is the reduced form of the aldotetrose erythrose. Draw erythritol.