Indicate whether the following statements apply to type 1 or type 2 diabetes:
(c) can be managed with diet and exercise

 Verified step by step guidance
Verified step by step guidance

Indicate whether the following statements apply to type 1 or type 2 diabetes:
(c) can be managed with diet and exercise
Identify the following carbohydrates as the ⍺ or β anomer:
(a)
Draw the ⍺ and β anomer of D-talose in pyranose ring form:
The sugar alcohol erythritol is often included in low-calorie sweeteners. It is 70% as sweet as table sugar. Erythritol is the reduced form of the aldotetrose erythrose. Draw erythritol.
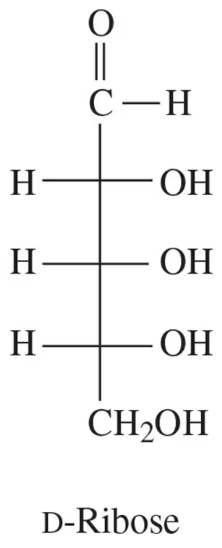
Pentoses also exist in a ring form, but they most commonly occur as furanose rings. D-Ribose exists in its furanose ring form in the nucleic acid RNA. Using the structure of D-ribose from Table 6.1, draw the furanose form of β-D-ribose.
Identify the following reactions as condensation or hydrolysis:
(a) two monosaccharides reacting to form a disaccharide