Identify the following carbohydrates as the ⍺ or β anomer:
(a)

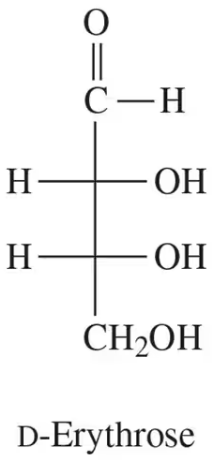
 Verified step by step guidance
Verified step by step guidance


Identify the following carbohydrates as the ⍺ or β anomer:
(a)
Draw the ⍺ and β anomer of D-talose in pyranose ring form:
The sugar alcohol ribitol is a component of the vitamin riboflavin and the energy transfer molecule FAD. Ribitol is formed when the monosaccharide ribose undergoes reduction at carbon 1. Draw the structure of ribitol.
Pentoses also exist in a ring form, but they most commonly occur as furanose rings. D-Ribose exists in its furanose ring form in the nucleic acid RNA. Using the structure of D-ribose from Table 6.1, draw the furanose form of β-D-ribose.
Identify the following reactions as condensation or hydrolysis:
(a) two monosaccharides reacting to form a disaccharide
Name the glycosidic bond present in mannobiose, shown in the following figure: