Will the following carbohydrates produce a positive Benedict's test?
a. D-glucose

 Verified step by step guidance
Verified step by step guidance

Will the following carbohydrates produce a positive Benedict's test?
a. D-glucose
Will the following carbohydrates produce a positive Benedict’s test?
b. lactose
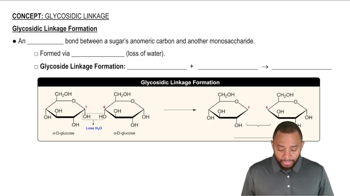
Draw the product of the following 1→4 condensation and name the glycosidic bond:
The glycosidic bond in a disaccharide was determined to be α(1→6). Hydrolysis of the disaccharide produced one galactose and one fructose. Draw the structure of the disaccharide.
Our bodies cannot digest cellulose because we lack the enzyme cellulase. Why is cellulose an important part of a healthy diet if we cannot digest it?
The shell of a shrimp is composed of chitin. If you eat a boiled shrimp without removing the shell, will your body break the shell down into its component sugars? Explain. (Hint: Compare chitin’s structure to that of amylose and cellulose.)