Textbook Question
Identify the following carbohydrates as the ⍺ or β anomer:
(b)
588
views

 Verified step by step guidance
Verified step by step guidance

Identify the following carbohydrates as the ⍺ or β anomer:
(b)
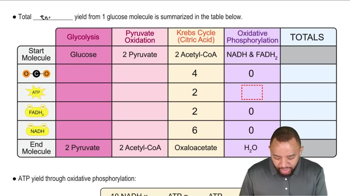
Draw the Fischer projection of the product of the oxidation of D-galactose at C1.
Draw the Fischer projection of the product of reduction reaction of D-galactose at C1.
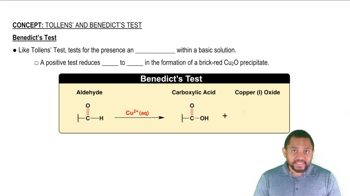
Will the following carbohydrates produce a positive Benedict’s test?
b. lactose
Draw the product of the following 1→4 condensation and name the glycosidic bond:
Isomaltose, a disaccharide formed during caramelization in cooking, contains two glucose units bonded ⍺(1→6). Draw the structure of isomaltose.