Will the following carbohydrates produce a positive Benedict’s test?
b. lactose

 Verified step by step guidance
Verified step by step guidance


Will the following carbohydrates produce a positive Benedict’s test?
b. lactose
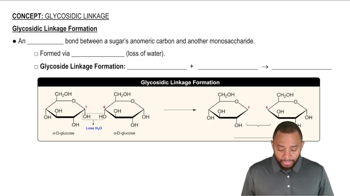
Draw the product of the following 1→4 condensation and name the glycosidic bond:
Isomaltose, a disaccharide formed during caramelization in cooking, contains two glucose units bonded ⍺(1→6). Draw the structure of isomaltose.
Our bodies cannot digest cellulose because we lack the enzyme cellulase. Why is cellulose an important part of a healthy diet if we cannot digest it?
The shell of a shrimp is composed of chitin. If you eat a boiled shrimp without removing the shell, will your body break the shell down into its component sugars? Explain. (Hint: Compare chitin’s structure to that of amylose and cellulose.)
Glycogen and amylopectin are both branched polymers of glucose. Read the descriptions of each in Section 6.6. Which molecule has a more compact structure? Explain.