The discovery in the 1960s that xenon and fluorine react to form a molecular compound was a surprise to most chemists, because it had been thought that noble gases could not form bonds.
a. Why was it thought that noble gases could not form bonds?

 Verified step by step guidance
Verified step by step guidance


The discovery in the 1960s that xenon and fluorine react to form a molecular compound was a surprise to most chemists, because it had been thought that noble gases could not form bonds.
a. Why was it thought that noble gases could not form bonds?
The following formulas are unlikely to be correct. What is wrong with each?
d. C2OS
Which of the following compounds contain ionic bonds? Which contain covalent bonds? Which contain coordinate covalent bonds? (A compound may contain more than one type of bond.)
a. BaCl2
The phosphonium ion, PH4+ is formed by reaction of phosphine, PH3, with an acid.
d. Explain why the ion has a +1 charge.
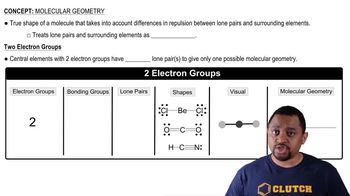
The sulfite ion (SO32–) and sulfur trioxide (SO3) have the same chemical formulas but different molecular geometries. Draw the Lewis dot structures and identify the molecular geometry of each.
Which of the following elements would you expect to form (iv) both covalent and ionic bonds? (More than one answer may apply; remember that some nonmetals can form ionic bonds with metals.) Explain your answers.
a. Oxygen
b. Potassium
c. Phosphorus
d. Iodine
e. Hydrogen
f. Cesium