Do the following reactions favor reactants or products at equilibrium? Give relative concentrations at equilibrium.
c. Fe2O3(s) + 3 CO(g) ⇌ 2 Fe(s) + 3 CO2(g) K (at 727 °C) = 24.2

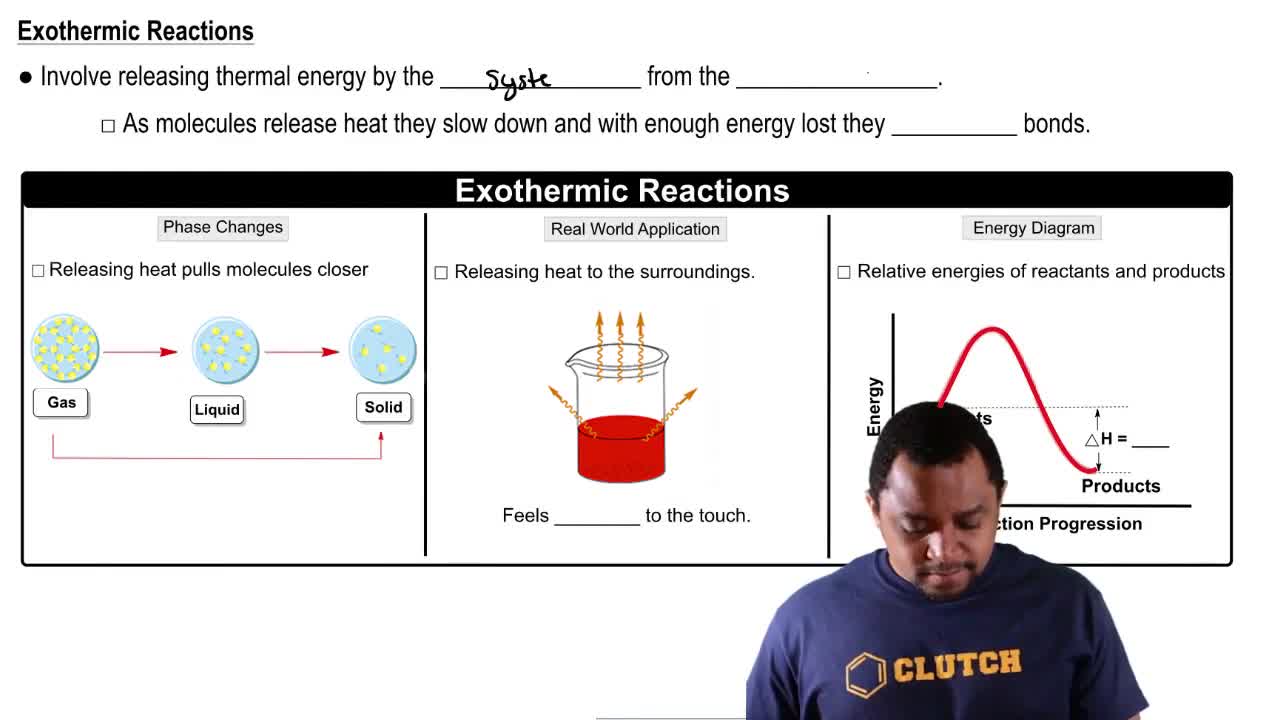
 Verified step by step guidance
Verified step by step guidance

Do the following reactions favor reactants or products at equilibrium? Give relative concentrations at equilibrium.
c. Fe2O3(s) + 3 CO(g) ⇌ 2 Fe(s) + 3 CO2(g) K (at 727 °C) = 24.2
The following diagrams represent two similar reactions that have achieved equilibrium:
<IMAGE>
a. Write the expression for the equilibrium constant for each reaction.
The following diagrams represent two similar reactions that have achieved equilibrium:
<IMAGE>
b. Calculate the value for the equilibrium constant for each reaction.
What effect do the listed changes have on the position of the equilibrium in the reaction of carbon with hydrogen?
C(s) + 2 H2(g) ⇌ CH4(g) ∆H = -18 kcal/mol (-75kJ/mol)
a. Increasing temperature
What effect do the listed changes have on the position of the equilibrium in the reaction of carbon with hydrogen?
C(s) + 2 H2(g) ⇌ CH4(g) ∆H = -18 kcal/mol (-75kJ/mol)
b. Increasing pressure by decreasing volume
What effect do the listed changes have on the position of the equilibrium in the reaction of carbon with hydrogen?
C(s) + 2 H2(g) ⇌ CH4(g) ∆H = -18 kcal/mol (-75kJ/mol)
c. Allowing CH4 to escape continuously from the reaction vessel