For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆H = –43 kcal/mol (–180 kJ/mol).
b. Under what conditions would you expect this process to be spontaneous?

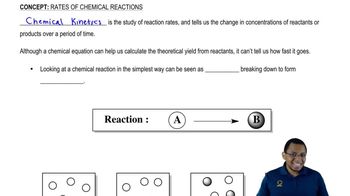
 Verified step by step guidance
Verified step by step guidance


For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆H = –43 kcal/mol (–180 kJ/mol).
b. Under what conditions would you expect this process to be spontaneous?
The following reaction is used in the industrial synthesis of polyvinyl chloride (PVC) polymer:
Cl2(g) + H2C=CH2(g) → ClCH2CH2Cl(l) ∆H = –52 kcal/mol (–218 kJ/mol)
a. Is ∆S positive or negative for this process?
Which reaction is faster, one with Eact = +10 kcal/mol(+41.8 kJ/mol) or one with Eact = +5 kcal/mol(+20.9 kJ/mol)? Explain.
What is a catalyst, and what effect does it have on the activation energy of a reaction?
If a catalyst changes the activation energy of a forward reaction from 28.0 kcal/mol to 23.0 kcal/mol, what effect does it have on the reverse reaction?
For the reaction C(s, diamond) → C(s, graphite), ∆G = -0.693 kcal/mol (-2.90 kJ/mol) at 25 °C.
a. According to this information, do diamonds spontaneously turn into graphite?