The change of state from liquid H2O to gaseous H2O has ∆H = +9.72 kcal/mol(+40.7 kJ/mol) and ∆S = -26.1 cal/(mol • K) [-109 J/(mol •K)].
b. What are the values of ∆H and ∆S (in kcal/mol and kJ/mol) for the change from gaseous to liquid H2O?

 Verified step by step guidance
Verified step by step guidance


The change of state from liquid H2O to gaseous H2O has ∆H = +9.72 kcal/mol(+40.7 kJ/mol) and ∆S = -26.1 cal/(mol • K) [-109 J/(mol •K)].
b. What are the values of ∆H and ∆S (in kcal/mol and kJ/mol) for the change from gaseous to liquid H2O?
Would you expect the boiling points to increase or decrease in the following series? Explain.
a. Kr, Ar, Ne
A local weather station reports the barometric pressure as 29.5 inHg (inches of Hg). Convert this pressure to torr and to atm.
Compare the ∆Hvap values for water, isopropyl alcohol, ether, and ammonia, and order them from lowest to highest. Explain the rank order based on intermolecular attractive forces.
Assume that you have a sample of gas at 350 K in a sealed container, as represented in part (a). Which of the drawings (b)–(d) represents the gas after the temperature is lowered from 350 K to 150 K and if the gas has a boiling point of 200 K? Which drawing represents the gas at 150 K if the gas has a boiling point of 100 K?
<IMAGE>
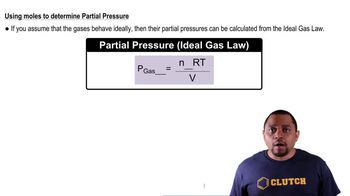
List four common units for measuring pressure.