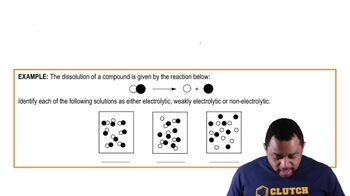
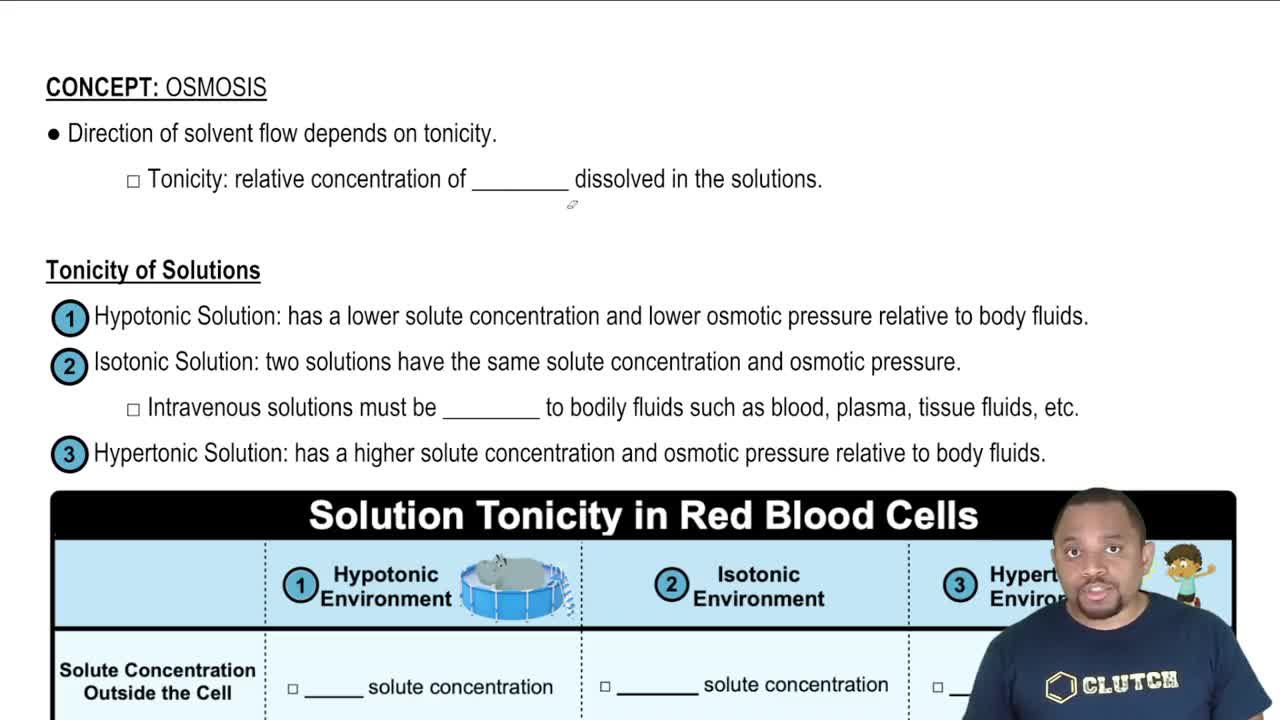
The diagram to the right shows plots of vapor pressure versus temperature for a solvent and a solution.
c. What is the approximate concentration of the solution in mol/kg, if 1 mol of solute particles raises the boiling point of 1 kg of solvent by 3.63 °C?
<IMAGE>