How many milliliters of 0.150 M BaCl2 are needed to react completely with 35.0 mL of 0.200 M Na2SO4? How many grams of BaSO4 will be formed?

Hyperbaric chambers, which provide high pressures (up to 6 atm) of either air or pure oxygen, are used to treat a variety of conditions, ranging from decompression sickness in deep-sea divers to carbon monoxide poisoning. Look up the solubility of O2, N2, CO, and CO2 in water at standard temperature and pressure (1 atm, 25 °C).
a. Explain the trends in relative solubility for these gases.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
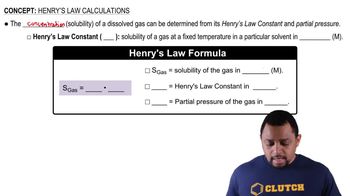
Gas Solubility

Henry's Law
Molecular Properties of Gases

Many compounds are only partially dissociated into ions in aqueous solution. Trichloroacetic acid (CCl3CO2H), for instance, is partially dissociated in water according to the equation
CCl3CO2H(aq) → H+(aq) + CCl3CO2⁻ aq)
For a solution prepared by dissolving 1.00 mol of trichloroacetic acid in 1.00 kg of water, 36.0% of the trichloroacetic acid dissociates to form H+ and CCl3CO2⁻ ions.
a. What is the total concentration of dissolved ions and molecules in 1 kg of water?
Many compounds are only partially dissociated into ions in aqueous solution. Trichloroacetic acid (CCl3CO2H), for instance, is partially dissociated in water according to the equation
CCl3CO2H(aq) → H+(aq) + CCl3CO2⁻ aq)
For a solution prepared by dissolving 1.00 mol of trichloroacetic acid in 1.00 kg of water, 36.0% of the trichloroacetic acid dissociates to form H+ and CCl3CO2⁻ ions.
b. What is the freezing point of this solution? (The freezing point of 1 kg of water is lowered 1.86 °C for each mole of solute particles.)
Hyperbaric chambers, which provide high pressures (up to 6 atm) of either air or pure oxygen, are used to treat a variety of conditions, ranging from decompression sickness in deep-sea divers to carbon monoxide poisoning. Look up the solubility of O2, N2, CO, and CO2 in water at standard temperature and pressure (1 atm, 25 °C).
b. Explain how elevated pressures in a hyperbaric chamber be used to treat decompression sickness (excess N2 in blood) and carbon monoxide poisoning.
Look up the composition of Ringer's solution used in the treatment of burns and wounds.
b. What is the osmolarity of the solution? Is it hypertonic, isotonic, or hypotonic with blood plasma (0.30 osmol)? Discuss possible medicinal reasons for the osmolarity of the solution.
To prevent accumulation of ice on roads and sidewalks, many municipalities (and home-owners) will apply de-icing compounds to 'melt' the ice by lowering the freezing point.
b. Some de-icing compositions include dyes or colored compounds called indicators. Why?
