What prefixes are used in naming the following?
a. A 1,3-disubstituted benzene
b. A 1,4-disubstituted benzene

 Verified step by step guidance
Verified step by step guidance
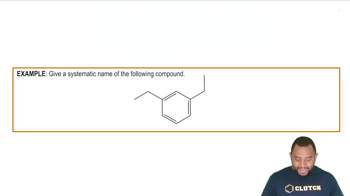


What prefixes are used in naming the following?
a. A 1,3-disubstituted benzene
b. A 1,4-disubstituted benzene
Write structural formulas for compounds that meet the following descriptions:
a. A 6-carbon alkene whose longest chain is 4 carbons in length (three possibilities)
Write structural formulas for compounds that meet the following descriptions:
b. An alkyne with 5 carbons total (three possibilities)
Write structural formulas for compounds that meet the following descriptions:
a. An alkene, C6H12, that cannot have cis–trans isomers and whose longest chain is 5 carbons long
Write structural formulas for compounds that meet the following descriptions:
b. An alkene with a chemical formula of C10H12, that has cis–trans isomers and contains a benzene ring.
Draw structures corresponding to the following IUPAC names:
a. trans-2-Pentene
b. trans-3,4-Dimethyl-3-hexene
c. 2-Methyl-1,3-butadiene
d. trans-3-Heptene