What two products result from the complete hydrolysis of this cyclic acetal?

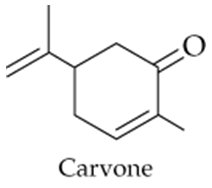
The compound carvone is responsible for the odor of spearmint. Identify the functional groups in carvone.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Functional Groups

Carvone Structure

Odor Compounds

Acetals and ketals are usually made by reaction of an aldehyde or ketone with two molecules of a monoalcohol. If an aldehyde or ketone reacts with one molecule of a dialcohol, however, a cyclic acetal or ketal results.
b. Draw the cyclic ketal formed when the hemiketal from part (a) reacts with the ―OH labeled in blue.
Aldosterone is a key steroid involved in controlling the sodium–potassium balance in the body. Identify the functional groups in aldosterone.
Can the alcohol (CH3)3COH be formed by the reduction of an aldehyde or ketone? Why or why not?
Many flavorings and perfumes are partially based on fragrant ketones, with far fewer being based on fragrant aldehydes. Why do you think ketones are used more frequently than aldehydes? See Section 15.5 for a clue.
Draw the structural formulas of the following compounds:
c. 2-Methoxy-2-methylpropane
