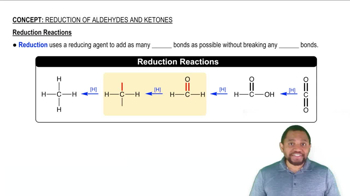
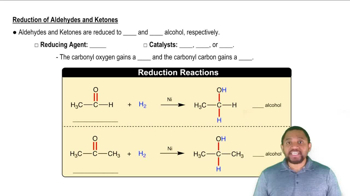
Acetals and ketals are usually made by reaction of an aldehyde or ketone with two molecules of a monoalcohol. If an aldehyde or ketone reacts with one molecule of a dialcohol, however, a cyclic acetal or ketal results.
b. Draw the cyclic ketal formed when the hemiketal from part (a) reacts with the ―OH labeled in blue.