Textbook Question
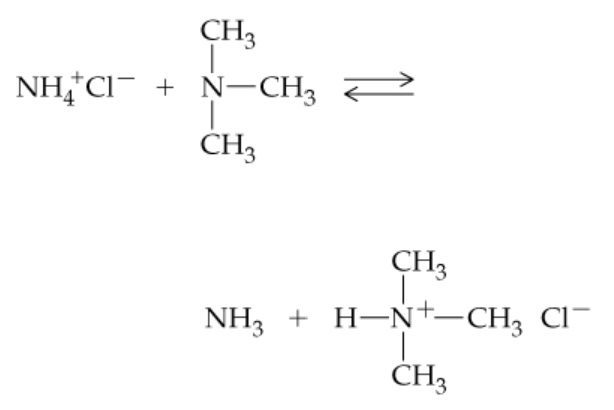
Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a.
788
views

 Verified step by step guidance
Verified step by step guidance


Complete the following equations. (Hint: Remember that a nitrogen with three groups bound to it has a lone pair and one with four does not)
a.
Many hair conditioners contain an ammonium salt such as the following to help prevent 'fly-away' hair. These ions will react with neither acid nor base. Provide a reason why.
Choline has the following structure. Do you think that this substance reacts with aqueous hydrochloric acid? If so, what is the product? If not, why not?
How do amines differ from analogous alcohols in (a) odor, (b) basicity, and (c) boiling point?
Name the following compounds:
a.
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
a.