In the following compound
b. Identify the phosphate anhydride linkage.

 Verified step by step guidance
Verified step by step guidance
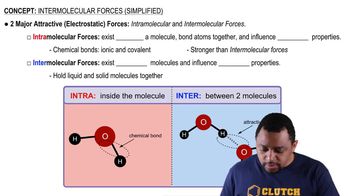


In the following compound
b. Identify the phosphate anhydride linkage.
Cyclic ribose nucleotide phosphates, such as cyclic AMP (cAMP), are important signaling agents in living cells; all have the general structure shown here. What kind of linkage holds the phosphate to the ribose (see arrows; ribose is highlighted in blue)?
What is the difference between a phosphate diester and an ester of a diphosphate? Give an example of each.
Mention at least two simple chemical tests by which you can distinguish between benzaldehyde and benzoic acid.
Write the formula of the triester formed from glycerol and stearic acid.
Each of the following materials has an ester that is responsible for its smell and/or flavor. Search the internet and determine what that ester is, draw its structure, and what carboxylic acid and alcohol are used to form it.
a. Juicy Fruit gum flavoring