Phenacetin (shown in the margin) was once used in headache remedies but is now banned because of its potential for causing kidney damage. (a) Identify all the functional groups present in phenacetin. (b) Draw the structures of the carboxylic acid and amine needed to prepare phenacetin.
Ch.17 Carboxylic Acids and Their Derivatives

Chapter 17, Problem 24a
What carboxylic acids and amines result from hydrolysis of the following amides?
a. 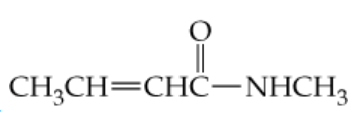
 Verified step by step guidance
Verified step by step guidance1
Identify the functional groups in the given amide structure. Amides consist of a carbonyl group (C=O) directly bonded to a nitrogen atom (N). The hydrolysis of an amide involves breaking the bond between the carbonyl carbon and the nitrogen atom.
Understand the hydrolysis reaction. Hydrolysis of an amide can occur under acidic or basic conditions. In acidic hydrolysis, the amide reacts with water and an acid catalyst to produce a carboxylic acid and an amine salt. In basic hydrolysis, the amide reacts with a base to produce a carboxylate ion and a free amine.
Determine the products of hydrolysis. The carbonyl carbon of the amide becomes part of the carboxylic acid (or carboxylate ion in basic hydrolysis), while the nitrogen atom and its attached groups form the amine (or amine salt in acidic hydrolysis).
Examine the specific structure of the given amide (from the image) to identify the R groups attached to the carbonyl carbon and the nitrogen atom. These R groups will determine the specific carboxylic acid and amine produced.
Write the balanced chemical equation for the hydrolysis reaction, showing the amide as the reactant and the carboxylic acid and amine (or their respective forms under acidic/basic conditions) as the products. Use the identified R groups to complete the equation.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrolysis of Amides
Hydrolysis of amides is a chemical reaction where an amide reacts with water, resulting in the formation of a carboxylic acid and an amine. This process typically requires an acid or base catalyst to facilitate the reaction. Understanding the structure of the amide is crucial, as it determines the specific carboxylic acid and amine produced.
Recommended video:
Guided course

Acidic Hydrolysis Concept 1
Carboxylic Acids
Carboxylic acids are organic compounds characterized by the presence of a carboxyl group (-COOH). They are known for their acidic properties and can be derived from the hydrolysis of amides. The specific carboxylic acid formed during hydrolysis depends on the alkyl or aryl group attached to the nitrogen in the amide.
Recommended video:
Guided course

Carboxylic Acid Reactions Example 1
Amines
Amines are organic compounds derived from ammonia by replacing one or more hydrogen atoms with alkyl or aryl groups. They can be classified as primary, secondary, or tertiary based on the number of carbon-containing groups attached to the nitrogen atom. The type of amine produced during the hydrolysis of an amide is influenced by the structure of the original amide.
Recommended video:
Guided course

Amine Classification Example 1
Related Practice
Textbook Question
684
views
Textbook Question
Draw the products you would obtain from acid-catalyzed hydrolysis of the following esters.
a. Isopropyl benzoate
628
views
Textbook Question
Draw the products you would obtain from acid-catalyzed hydrolysis of the following esters.
b.
708
views
Textbook Question
What carboxylic acids and amines result from hydrolysis of the following amides?
b. N,N-Dimethyl-p-nitrobenzamide
684
views
Textbook Question
Give the structure of the repeating units in the polymers that are formed in the reactions of the following compounds.
a.
647
views
Textbook Question
Write the formula for the phosphate monoester formed from isopropyl alcohol and phosphoric acid.
31
views
