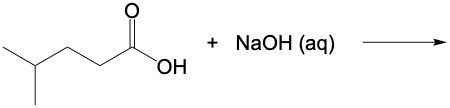
Carboxylic acids, such as ethanoic acid, are classified as weak acids and participate in acid-base reactions. In these reactions, a base interacts with the carboxylic acid, resulting in the removal of a hydrogen ion (H+). This process transforms the carboxylic acid into its conjugate base, known as a carboxylate anion.
For instance, when ethanoic acid (CH3COOH) reacts with a base, it loses an H+ ion, leading to the formation of ethanoate (CH3COO-). The naming convention changes from "oic acid" in the carboxylic acid form to "oate" in the carboxylate anion form. This distinction is crucial in understanding the relationship between acids and their conjugate bases in chemical reactions.
In summary, the reaction of a carboxylic acid with a base results in the formation of a carboxylate anion, highlighting the fundamental principles of acid-base chemistry.