Which amino acid is hydrophilic (dissolves in aqueous solutions)? Why?
a. isoleucine
b. phenylalanine
c. aspartic acid

 Verified step by step guidance
Verified step by step guidance

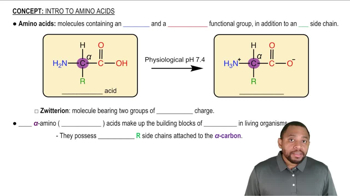

Which amino acid is hydrophilic (dissolves in aqueous solutions)? Why?
a. isoleucine
b. phenylalanine
c. aspartic acid
Is serine chiral? Draw serine and identify the chiral atom. Explain why serine is chiral.
Two of the 20 common amino acids have two chiral carbon atoms in their structures. Identify these amino acids and their chiral carbon atoms.
Valine is an amino acid with a nonpolar side chain, and serine is an amino acid with a polar side chain. Draw the two dipeptides that can be formed by these two amino acids. Identify the peptide bond.
Tripeptides are composed of three amino acids linked by peptide bonds. Given a set of amino acids, you can make several different tripeptides.
a. Use the three-letter shorthand notations to name all the tripeptides that can be made from serine, tyrosine, and glycine. Each amino acid will be used once in each tripeptide.
Using three-letter abbreviations, show the six tripeptides that contain isoleucine, arginine, and valine.