How do the following interactions help to stabilize the tertiary and quaternary structure of a protein? Give an example of a pair of amino acids that could give rise to each interaction.
b. Disulfide bonds

 Verified step by step guidance
Verified step by step guidance
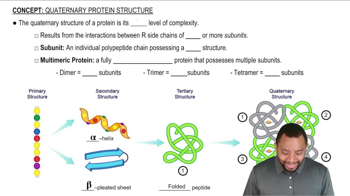


How do the following interactions help to stabilize the tertiary and quaternary structure of a protein? Give an example of a pair of amino acids that could give rise to each interaction.
b. Disulfide bonds
Give an example of a protein that has quaternary structure. How many polypeptide chains are present in this protein?
Explain how a protein is denatured by the following:
a. Heat
Explain how a protein is denatured by the following:
c. Organic solvents
Fresh pineapple cannot be used in gelatin desserts because it contains an enzyme that hydrolyzes the proteins in gelatin, destroying the gelling action. Canned pineapple can be added to gelatin with no problem. Why?
As a chef, you prepare a wide variety of foods daily. The following dishes all contain protein. What method (if any) has been used to denature the protein present in each food?
a. Charcoal-grilled steak