How are amylose and amylopectin similar to each other, and how are they different from each other?
Ch.20 Carbohydrates

Chapter 20, Problem 76
D-Fructose can form a six-membered cyclic hemiacetal as well as the more prevalent five-membered cyclic form. Draw the α isomer of D-fructose in the six-membered ring.
 Verified step by step guidance
Verified step by step guidance1
Understand that d-fructose is a ketohexose, meaning it contains six carbons and a ketone functional group. The cyclic forms are created when the ketone group reacts with a hydroxyl group on the molecule to form a hemiacetal.
Identify the six-membered cyclic form of d-fructose. This is called a pyranose form, where the ketone group on carbon 2 reacts with the hydroxyl group on carbon 6 to form a six-membered ring.
Draw the six-membered ring structure. Place oxygen as one of the ring members and arrange the remaining carbons (C2 through C6) and their substituents around the ring. Ensure the ring closure occurs between C2 and C6.
Determine the α isomer. In the α isomer, the hydroxyl group attached to the anomeric carbon (C2 in this case) is positioned *down* (opposite to the CH2OH group attached to C5) in the Haworth projection.
Complete the structure by adding the remaining hydroxyl groups and hydrogen atoms to the appropriate carbons, ensuring the stereochemistry matches that of d-fructose. Verify that the configuration of the chiral centers (C3, C4, and C5) corresponds to the d-fructose configuration.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
8mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Cyclic Hemiacetal Formation
Cyclic hemiacetals are formed when a carbonyl group (aldehyde or ketone) reacts with an alcohol, resulting in a ring structure. In the case of d-fructose, the ketone group at C2 reacts with the hydroxyl group on C5, leading to the formation of a six-membered ring. This reaction is crucial for understanding the structural variations of sugars.
Recommended video:
Guided course
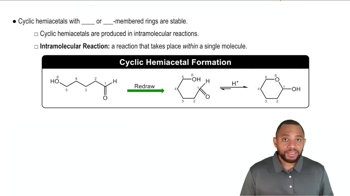
Cyclic Hemiacetals Concept 2
Anomeric Carbon
The anomeric carbon is the carbon atom in a sugar that was originally part of the carbonyl group and becomes a new chiral center upon cyclization. In d-fructose, the anomeric carbon is C2 in the six-membered ring. The configuration at this carbon determines whether the sugar is in the α or β form, which is essential for distinguishing between different isomers.
Recommended video:
Guided course
Amino Acid Catabolism: Carbon Atoms Concept 2
Furanose vs. Pyranose Forms
Sugars can exist in different cyclic forms, primarily furanose (five-membered) and pyranose (six-membered) rings. d-Fructose predominantly exists as a furanose, but it can also form a pyranose structure. Understanding these forms is important for grasping the reactivity and biological roles of sugars in various biochemical processes.
Recommended video:
Guided course

Periodic Table: Elemental Forms (Simplified) Concept 1
Related Practice
Textbook Question
588
views
Textbook Question
Trehalose, a disaccharide found in the blood of insects, has the following structure. What simple sugars would you obtain on hydrolysis of trehalose? (Hint: Rotate one of the rings in your head or redraw it rotated.)
1111
views
Textbook Question
Are the α and β forms of the disaccharide lactose enantiomers of each other? Why or why not?
998
views
Textbook Question
Describe the differences between mono-, di-, and polysaccharides.
1617
views
Textbook Question
Name a naturally occurring carbohydrate and its source for each type of carbohydrate listed in Problem 20.83.
658
views
Textbook Question
Carbohydrates provide 4 kcal per gram. If a person eats 200 g per day of digestible carbohydrates, what percentage of a 2000 kcal daily diet would be digestible carbohydrate?
743
views
