Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
b. What class of enzymes carry out these reactions?

 Verified step by step guidance
Verified step by step guidance

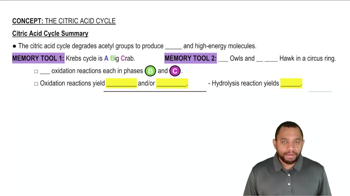

Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
b. What class of enzymes carry out these reactions?
Why, do you suppose, the coenzyme for the reaction in the citric acid cycle that is catalyzed by succinate dehydrogenase is FAD and not NAD+?
Identify the participants in the citric acid cycle that contain alcohol groups. Identify these groups as primary, secondary, or tertiary alcohols.
The reduced coenzymes NADH and FADH2 are oxidized in the ETS. What is the final electron acceptor of the ETS? What is the function of the H+ ion in ATP synthesis?
Each of these reactions is involved in one of the four stages of metabolism shown in Figure 21.4. Identify the stage in which each reaction occurs.
<IMAGE>
a. Hydrolysis of starch to produce glucose
Each of these reactions is involved in one of the four stages of metabolism shown in Figure 21.4. Identify the stage in which each reaction occurs.
<IMAGE>
b. Oxidation of NADH coupled with synthesis of ATP