Which of the following is found in the coenzyme FAD?
a. Two heterocyclic rings
b. ADP
c. A substituted benzene ring
d. A phosphate anhydride bond

 Verified step by step guidance
Verified step by step guidance

Which of the following is found in the coenzyme FAD?
a. Two heterocyclic rings
b. ADP
c. A substituted benzene ring
d. A phosphate anhydride bond
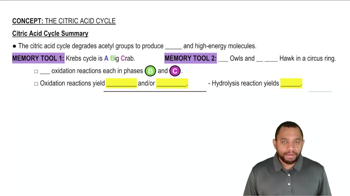
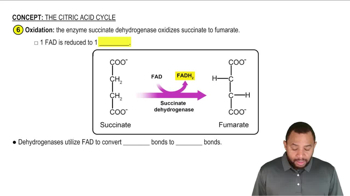
Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
a. Draw the structures of the reactants in steps 3, 6, and 8, and indicate which hydrogen atoms are removed in these reactions.
Look ahead to Figure 21.8 for the citric acid cycle.
<IMAGE>
b. What class of enzymes carry out these reactions?
Identify the participants in the citric acid cycle that contain alcohol groups. Identify these groups as primary, secondary, or tertiary alcohols.
Which of the reactants in the citric acid cycle have two chiral carbon atoms?
The reduced coenzymes NADH and FADH2 are oxidized in the ETS. What is the final electron acceptor of the ETS? What is the function of the H+ ion in ATP synthesis?