Textbook Question
What is the difference between digestion and metabolism?
1519
views

 Verified step by step guidance
Verified step by step guidance


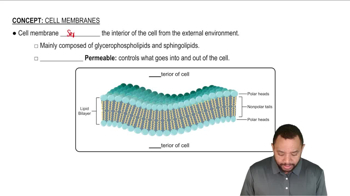
What is the difference between digestion and metabolism?
Arrange the following events in the order in which they occur in a catabolic process: electron transport, digestion, oxidative phosphorylation, citric acid cycle.
What key metabolic intermediate is formed from the catabolism of all three major classes of foods: carbohydrates, lipids, and proteins?
What general kind of chemical reaction does ATP participate in?
What does it mean when we say that two reactions are coupled?
Write the reaction for the hydrolysis of 1,3-bisphosphoglycerate coupled to the phosphorylation of ADP using the curved-arrow symbolism.