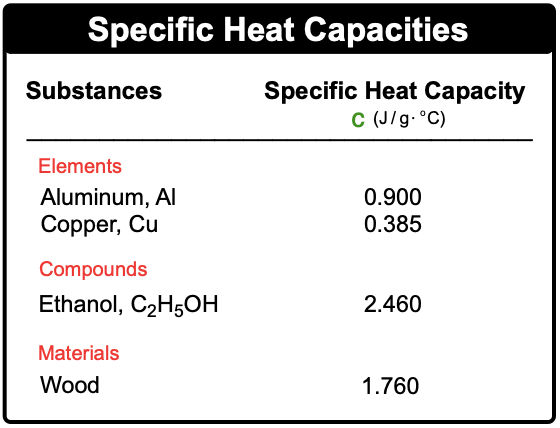
Heat capacity is a fundamental concept in thermodynamics that describes how a substance responds to the application of heat. When heat is added to an object, its temperature increases, demonstrating a direct relationship between the amount of heat applied and the resulting temperature change. This relationship can be expressed mathematically as:
q ∝ ΔT
In this equation, q represents the heat added to the substance, and ΔT is the change in temperature. The proportionality indicates that as the heat (q) increases, the temperature change (ΔT) also increases, assuming the substance's heat capacity remains constant. Understanding this relationship is crucial for predicting how different materials will respond to heat, which has practical applications in various scientific and engineering fields.