If all the solute is dissolved in diagram 1, how would heating or cooling the solution cause each of the following changes?
a. 2 to 3
<IMAGE>

 Verified step by step guidance
Verified step by step guidance
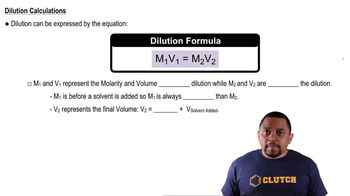


If all the solute is dissolved in diagram 1, how would heating or cooling the solution cause each of the following changes?
a. 2 to 3
<IMAGE>
Select the diagram (1, 2, or 3) that represents the solution formed by a solute represented by?
<IMAGE>
that is a (9.2)
a. nonelectrolyte
<IMAGE>
Select the diagram (1, 2, or 3) that represents the solution formed by a solute represented by?
<IMAGE>
that is a (9.2)
c. strong electrolyte
<IMAGE>
A pickle is made by soaking a cucumber in brine, a salt-water solution. What makes the smooth cucumber become wrinkled like a prune?
A semipermeable membrane separates two compartments, A and B. If the levels in A and B are equal initially, select the diagram that illustrates the final levels in a to d:
<IMAGE>
Select the diagram (1, 2, or 3) that represents the shape of a red blood cell when placed in each of the following a to e: (9.6)
<IMAGE>
a. 0.9% (m/v) NaCl solution