Under special conditions, sulfur reacts with anhydrous liquid ammonia to form a binary compound of sulfur and nitrogen. The compound is found to consist of 69.6% S and 30.4% N. Measurements of its molecular mass yield a value of 184.3 g/mol. The compound occasionally detonates on being struck or when heated rapidly. The sulfur and nitrogen atoms of the molecule are joined in a ring. All the bonds in the ring are of the same length. (b) Write Lewis structures for the molecule, based on the information you are given. (Hint: You should find a relatively small number of dominant Lewis structures.)

Trifluoroacetic acid has the chemical formula CF3CO2H. It is a colorless liquid that has a density of 1.489 g/mL (a) Trifluoroacetic acid contains one CF3 unit and is connected to the other C atom which bonds with both O’s. Draw the Lewis structure for trifluoroacetic acid.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
Lewis Structures

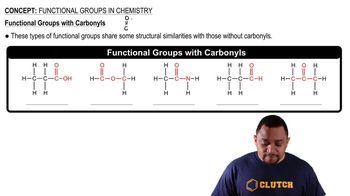
Functional Groups
Molecular Geometry

A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent:
(b) How many P-P bonds are there in the molecule?
A common form of elemental phosphorus is the tetrahedral P4 molecule, where all four phosphorus atoms are equivalent:
Draw a Lewis structure for a linear P4 molecule that satisfies the octet rule. Does this molecule have resonance structures?
Trifluoroacetic acid has the chemical formula CF3CO2H. It is a colorless liquid that has a density of 1.489 g/mL. (b) Trifluoroacetic acid can react with NaOH in aqueous solution to produce the trifluoroacetate ion, CF3COO2. Write the balanced chemical equation for this reaction.
Trifluoroacetic acid has the chemical formula CF3CO2H. It is a colorless liquid that has a density of 1.489 g/mL. (d) How many milliliters of a 0.500 M solution of NaOH would it take to neutralize 10.5 mL of trifluoroacetic acid?
