A three-gene system of additive genes (A, B, and C) controls plant height. Each gene has two alleles (A and a, B and b, and C and c). There is dominance among the alleles of each gene, with alleles A, B, and C dominant over a, b, and c. Under this scheme, the dominant genotype for a gene contributes 10 cm to height potential, and the recessive genotype contributes 4 cm. What are the phenotypes and proportions of each phenotype among the F₂?

You have cloned a gene for an enzyme that degrades lipids in a bacterium that normally lives in cold temperatures. You wish to transfer this gene into E. coli to produce industrial amounts of enzyme for use in laundry detergent.
You have managed to produce transgenic E. coli expressing mRNA of your gene, but only a low level of protein is produced. Why might this be so? How could you overcome this problem?
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
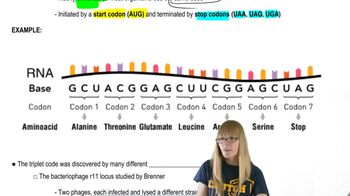
Gene Expression Regulation

Codon Optimization
Post-Translational Modifications

The RAS gene encodes a signaling protein that hydrolyzes GTP to GDP. When bound by GDP, the RAS protein is inactive, whereas when bound by GTP, RAS protein activates a target protein, resulting in stimulation of cells to actively grow and divide. As shown in the accompanying sequence, a single base-pair mutation results in a mutant protein that is constitutively active, leading to continual promotion of cell proliferation. Such mutations play a role in the formation of cancer. You have cloned the wild-type version of the mouse RAS gene and wish to create a mutant form to study its biological activity in vitro and in transgenic mice. Outline how you would proceed.
You have cloned a gene for an enzyme that degrades lipids in a bacterium that normally lives in cold temperatures. You wish to transfer this gene into E. coli to produce industrial amounts of enzyme for use in laundry detergent.
How would you accomplish this?
Describe how having the Cas9 gene at a genomic locus unlinked to the guide RNA and target site locus in an engineered gene drive system could slow the propagation of the gene drive allele in a population into which a small number of individuals carrying both the gene drive allele and the Cas9 locus are released.
Would a gene drive system spread rapidly through a population in a species that tends to self-mate (e.g., Arabidopsis, C. elegans)? In a species in which the breeding cycle is slow (e.g., humans)?
