Give the name and three-letter abbreviation for the amino acid described by each of the following:
a. the polar amino acid with a benzene ring in its side chain

 Verified step by step guidance
Verified step by step guidance

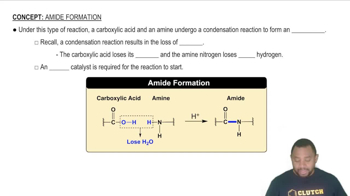
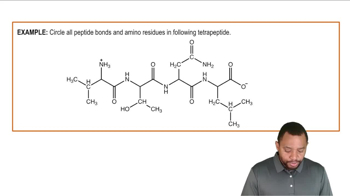
Give the name and three-letter abbreviation for the amino acid described by each of the following:
a. the polar amino acid with a benzene ring in its side chain
Give the name and three-letter abbreviation for the amino acid described by each of the following:
c. the polar amino acid with a sulfur atom in its side chain
Aspartame, which is commonly known as NutraSweet™, contains the following dipeptide:
d. Draw the structure of the isomer of this dipeptide where the C-terminal and N-terminal amino acids are switched.
Consider the amino acids glycine, proline, and lysine.
b. Using three-letter abbreviations for the amino acids, give the sequence for each of the possible tripeptides.
Would you expect to find this segment at the center or on the surface of a globular protein? Why?
Name the covalent bond that helps to stabilize the tertiary structure of a protein.