Aspartame, which is commonly known as NutraSweet™, contains the following dipeptide:
d. Draw the structure of the isomer of this dipeptide where the C-terminal and N-terminal amino acids are switched.

 Verified step by step guidance
Verified step by step guidance
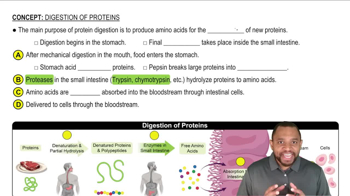

Aspartame, which is commonly known as NutraSweet™, contains the following dipeptide:
d. Draw the structure of the isomer of this dipeptide where the C-terminal and N-terminal amino acids are switched.
Draw the structure of the possible dipeptides formed from one alanine combining with one cysteine.
Consider the amino acids glycine, proline, and lysine.
b. Using three-letter abbreviations for the amino acids, give the sequence for each of the possible tripeptides.
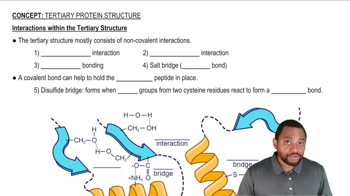
Name the covalent bond that helps to stabilize the tertiary structure of a protein.
Identify some differences between the following pairs:
b. complete and incomplete proteins
Identify the level of protein structure associated with each of the following:
b. hydrogen bonding between backbone atoms