Textbook Question
Would you expect to find this segment at the center or on the surface of a globular protein? Why?
921
views

 Verified step by step guidance
Verified step by step guidance


Would you expect to find this segment at the center or on the surface of a globular protein? Why?
Name the covalent bond that helps to stabilize the tertiary structure of a protein.
Identify some differences between the following pairs:
b. complete and incomplete proteins
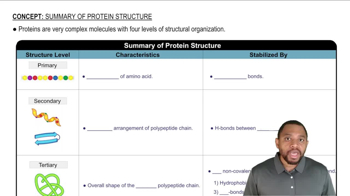
Identify the level of protein structure associated with each of the following:
d. intermolecular forces between R groups
Identify the level of protein structure associated with each of the following:
b. disulfide bridge
Identify the level of protein structure associated with each of the following:
d. salt bridges between polypeptides