Textbook Question
List the names and abbreviations of the four nucleotides in RNA.
1077
views

 Verified step by step guidance
Verified step by step guidance



List the names and abbreviations of the four nucleotides in RNA.
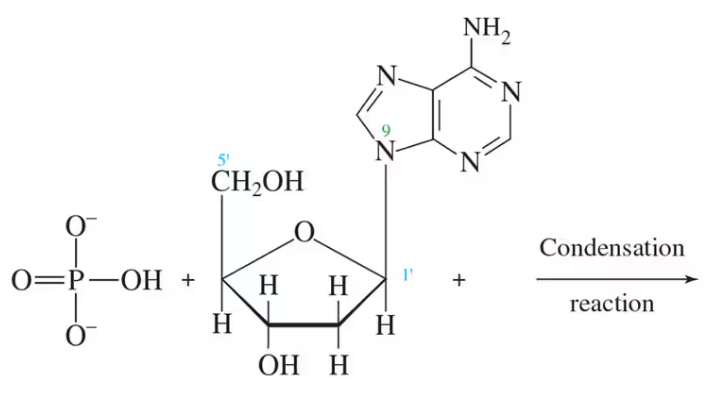
Provide the products for each of the following condensation reactions:
a.
Provide the products for each of the following condensation reactions:
a.
What is the name of the bond that joins nucleotides in a nucleic acid?
Describe the differences in the two ends of a nucleic acid.
Draw the dinucleotide AT that would be found in DNA. Label the 5' and 3' ends of your structure. Identify the phosphodiester bond.