Glucose, a form of sugar, has the formula C6H12O6. Which elements are included in this compound, and how many atoms of each are present?
Ch.1 Matter and Measurements

Chapter 1, Problem 56
Give the full name of the following units:
a. cc
b. dm
c. mm
d. nL
e. mg
f. m3
 Verified step by step guidance
Verified step by step guidance1
Step 1: Understand that the question is asking for the full names of the given units, which are abbreviations commonly used in scientific measurements.
Step 2: For 'cc', recognize that it stands for 'cubic centimeter', a unit of volume equivalent to 1 milliliter (mL).
Step 3: For 'dm', identify it as 'decimeter', which is a unit of length equal to one-tenth of a meter (10⁻¹ m).
Step 4: For 'mm', determine that it represents 'millimeter', a unit of length equal to one-thousandth of a meter (10⁻³ m).
Step 5: For 'nL', 'mg', and 'm³', recognize that they stand for 'nanoliter' (10⁻⁹ liters), 'milligram' (10⁻³ grams), and 'cubic meter' (a unit of volume), respectively.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Centicenti (cc)
The unit 'cc' stands for cubic centimeter, which is a volume measurement equivalent to one milliliter. It is commonly used in fields such as medicine and science to quantify liquid volumes. One cubic centimeter is defined as the volume of a cube with sides of one centimeter.
Recommended video:
Guided course

Conversion Factors (Simplified) Concept 2
Decimeter (dm)
The unit 'dm' refers to decimeter, a metric unit of length equal to one-tenth of a meter. It is often used in contexts where measurements are needed in a more manageable scale than meters, such as in education and everyday measurements. One decimeter is equivalent to 10 centimeters.
Recommended video:
Guided course

Density
Milligram (mg)
The unit 'mg' stands for milligram, which is a metric unit of mass equal to one-thousandth of a gram. It is frequently used in fields like pharmacology and nutrition to measure small quantities of substances. Understanding milligrams is essential for accurate dosing and nutritional information.
Recommended video:
Guided course
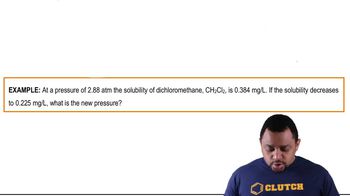
Henry's Law Calculations Example 2
Related Practice
Textbook Question
2024
views
Textbook Question
Write the formula for ibuprofen: 13 carbons, 18 hydrogens, and 2 oxygens. What are the common uses of ibuprofen?
2019
views
Textbook Question
What is the difference between a physical quantity and a number?
2893
views
Textbook Question
The white blood cell concentration in normal blood is approximately 12,000 cells/mm3 of blood. How many white blood cells does a normal adult with 5 L of blood have? Express the answer in scientific notation.
1652
views
1
rank
Textbook Question
Calculate the specific heat of copper if it takes 23 cal (96 J) to heat a 5.0 g sample from 25 °C to 75 °C.
1674
views
Textbook Question
The specific heat of fat is 0.45 cal/(g ⋅ °C) (1.9 J/g °C) and the density of fat is 0.94 g/cm3. How much energy (in calories and joules) is needed to heat 10 cm3 of fat from room temperature (25 °C) to its melting point (35 °C)?
2550
views
1
rank
