Classify the following liquid mixtures as heterogeneous or homogeneous. Further classify each homogeneous mixture as a solution or colloid.
c. Hand lotion

 Verified step by step guidance
Verified step by step guidance
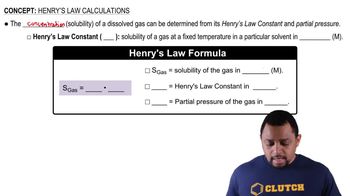

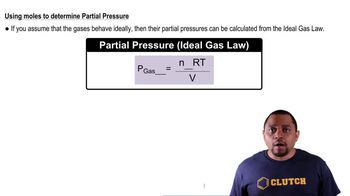
Classify the following liquid mixtures as heterogeneous or homogeneous. Further classify each homogeneous mixture as a solution or colloid.
c. Hand lotion
Which of the following pairs of substances would you expect to form solutions?
a. CCl4 and water
b. Benzene (C6H6) and MgSO4
c. Hexane (C6H14) and heptane (C7H16)
d. Ethyl alcohol (C2H5OH) and heptanol (C7H15OH)
A solution is prepared by dissolving 12.5 g of KBr in 20 mL of water at 60 °C (see Figure 9.3). Is this solution saturated, unsaturated, or supersaturated? What will happen if the solution is cooled to 10 °C?
<IMAGE>
The maximum amounts of lead and copper allowed in drinking water are 0.015 mg/kg for lead and 1.3 mg/kg for copper. Express these values in parts per million, and tell the maximum amount of each (in grams) allowed in 100 g of water.
The concentration of cholesterol (C27H46O) in blood is approximately 5.0 mM. How many grams of cholesterol are in 250 mL of blood?