Textbook Question
What are the IUPAC names of the following alkanes?
a.
1753
views

 Verified step by step guidance
Verified step by step guidance
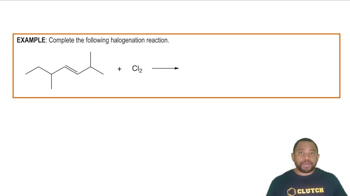

What are the IUPAC names of the following alkanes?
a.
Draw both condensed and line structures corresponding to the following IUPAC names and label each carbon as primary, secondary, tertiary, or quaternary.
c. 2,2,4-Trimethylpentane
Draw and name alkanes that meet the following descriptions:
a. A 5-carbon alkane with a tertiary carbon atom
What are the IUPAC names of the following cycloalkanes? Remember to assign priority to the attached groups alphabetically.
b.
What is wrong with the following names? It will be helpful to draw the structures as named before making your decision.
c. 1-Ethyl-2-methyl-3-ethylcyclopentane
Convert the following models into line drawings (back = C; white = H; blue = N):
a.