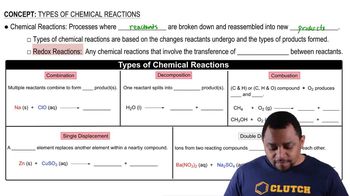
Textbook Question
Give systematic names for the following alkyl halides:
a.
b.
1392
views

 Verified step by step guidance
Verified step by step guidance

Give systematic names for the following alkyl halides:
a.
b.
2-Aminopropane is an achiral molecule, but 2-aminobutane is chiral. Explain.
Which of the following molecules are chiral? (Hint: Draw each molecule and analyze it as illustrated in Worked Example 14.2.)
a. 3-Chloropentane
b. 2-Chloropentane
c.
The compound pictured here is a thiol. (a) Draw its line structure, and (b) draw the structure of the disulfide formed when it is treated with an oxidizing agent (yellow = S).
<IMAGE>
Draw structures corresponding to the following names:
a. 2,4-Dimethyl-2-heptanol
Draw structures corresponding to the following names:
b. 2,2-Diethylcyclohexanol