Textbook Question
Give systematic names for the following carboxylic acids:
d.
535
views

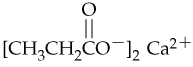
 Verified step by step guidance
Verified step by step guidance



Give systematic names for the following carboxylic acids:
d.
Give systematic names for the following carboxylic acids:
d. CH3(CH2)5COOH
Give systematic names for the following carboxylic acid salts:
a.
Draw structures corresponding to the following names:
c. 3,3-Dimethyl-4-phenylpentanoic acid
Fumaric acid is a metabolic intermediate that has the systematic name trans-2-butenedioic acid. Draw its structure.
What is the formula for the diammonium salt of fumaric acid?