Textbook Question
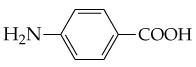
Write the equation for the ionization of hexanoic acid in water at pH 7.4.
865
views

 Verified step by step guidance
Verified step by step guidance


Write the equation for the ionization of hexanoic acid in water at pH 7.4.
Suppose you have a sample of benzoic acid dissolved in water.
b. Now assume that aqueous NaOH is added to the benzoic acid solution until pH 12 is reached. Draw the structure of the major organic species present.
Suppose you have a sample of benzoic acid dissolved in water.
c. Finally, assume that aqueous HCl is added to the solution from (b) until pH 2 is reached. Draw the structure of the major organic species present.
Give systematic names for the following carboxylic acids:
d. CH3(CH2)5COOH
Give systematic names for the following carboxylic acid salts:
a.
Give systematic names for the following carboxylic acid salts:
c.