Textbook Question
Write the reaction for the hydrolysis of 1,3-bisphosphoglycerate coupled to the phosphorylation of ADP using the curved-arrow symbolism.
863
views

 Verified step by step guidance
Verified step by step guidance
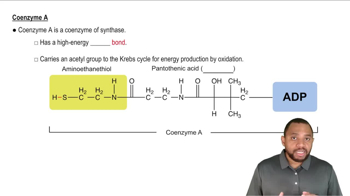

Write the reaction for the hydrolysis of 1,3-bisphosphoglycerate coupled to the phosphorylation of ADP using the curved-arrow symbolism.
FAD is a coenzyme for dehydrogenation.
a. When a molecule is dehydrogenated, is FAD oxidized or reduced?
FAD is a coenzyme for dehydrogenation.
b. Is FAD an oxidizing agent or a reducing agent?
What is the purpose of the citric acid cycle?
Where in the cell does the citric acid cycle take place?
What is the final fate of the carbons in acetyl-CoA after several turns of the citric acid cycle?