Textbook Question
What are the ultimate products of the electron-transport chain?
1125
views

 Verified step by step guidance
Verified step by step guidance
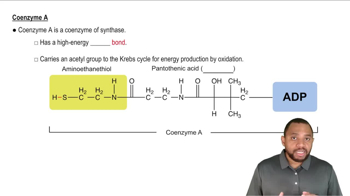
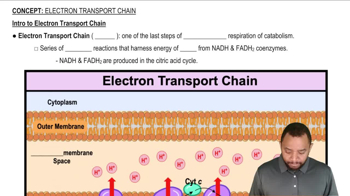
What are the ultimate products of the electron-transport chain?
What do the following abbreviations stand for?
b. CoQ
What do the following abbreviations stand for?
c. NADH/H+
What would happen to the citric acid cycle if NADH and FADH2 were not reoxidized?
What does the term “oxidative phosphorylation” mean? What is substrate-level phosphorylation? Are these processes the same? Explain.
In oxidative phosphorylation, what is oxidized and what is phosphorylated?