Textbook Question
List the four biological molecules commonly found in living organisms.
2000
views

 Belk, Maier 6th Edition
Belk, Maier 6th Edition Ch. 2 - Science Fiction, Bad Science, and Pseudoscience
Ch. 2 - Science Fiction, Bad Science, and Pseudoscience Problem 4
Problem 4 Verified step by step guidance
Verified step by step guidance
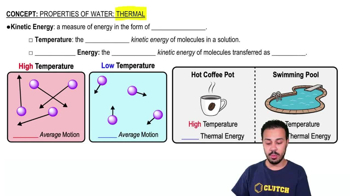


List the structural features in a prokaryotic cell.
Add labels to the figure that follows, which illustrates the subatomic particles associated with a carbon atom.
Electrons ________.
a. Are negatively charged.
b. Along with neutrons make up the nucleus.
c. Are attracted to the negatively charged nucleus.
d. Are not involved in ionic bonds.
e. All of the above are true.
Which of the following terms is least like the others?
a. Monosaccharide
b. Phospholipid
c. Fat
d. Steroid
e. Lipid
Different proteins are composed of different sequences of ________.
a. Sugars
b. Lipids
c. Fats
d. Amino acids
e. Carbohydrates