Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (a) Do you expect the element to be a gas, liquid, or solid at room temperature?

(a) Why was the term inert gases dropped? (b) What discovery triggered this change in name? (c) What name is applied to the group 18 elements now?
 Verified step by step guidance
Verified step by step guidanceKey Concepts
Inert Gases vs. Noble Gases

Discovery of Chemical Reactivity
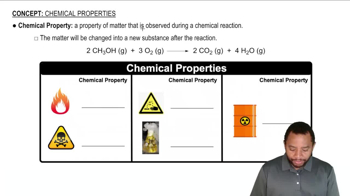
Group 18 Elements

Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (b) Would you expect At to be a metal, nonmetal, or metalloid? Explain.
Little is known about the properties of astatine, At, because of its rarity and high radioactivity. Nevertheless, it is possible for us to make many predictions about its properties. (c) What is the chemical formula of the compound it forms with Na?
Write a balanced equation for the reaction that occurs in each of the following cases: (a) White phorphrous, P4(s), reacts with chlorine gas. (b) Sodium metal reacts with water. (c) Sulfur reacts with hydrogen gas.
Write a balanced equation for the reaction that occurs in each of the following cases: (d) Fluorine reacts with water.
