Textbook Question
Write a balanced equation for the reaction that occurs in each of the following cases: (a) White phorphrous, P4(s), reacts with chlorine gas. (b) Sodium metal reacts with water. (c) Sulfur reacts with hydrogen gas.
697
views

 Verified step by step guidance
Verified step by step guidance
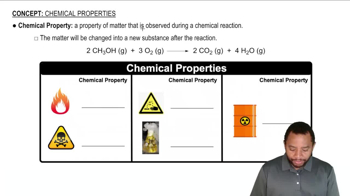


Write a balanced equation for the reaction that occurs in each of the following cases: (a) White phorphrous, P4(s), reacts with chlorine gas. (b) Sodium metal reacts with water. (c) Sulfur reacts with hydrogen gas.
Consider the stable elements through lead (Z = 82). In how many instances are the atomic weights of the elements out of order relative to the atomic numbers of the elements?
Figure 7.4 shows the radial probability distribution functions for the 2s orbitals and 2p orbitals. (a) Which orbital, 2s or 2p, has more electron density close to the nucleus?