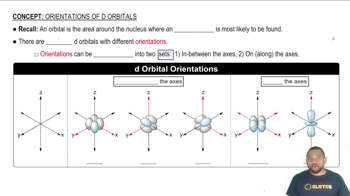
2. The atomic orbitals are grouped into different energy levels or shells, and each shell is divided into subshells. The first energy level has one subshell (s), the second and third levels have two subshells (s and p), the fourth and fifth levels have three subshells (s, p, and d), and the sixth and seventh levels have four subshells (s, p, d, and f).

 Verified step by step guidance
Verified step by step guidance