Textbook Question
A glucose solution contains 55.8 g of glucose (C6H12O6) in 455 g of water. Determine the freezing point and boiling point of the solution.
3181
views

 Verified step by step guidance
Verified step by step guidance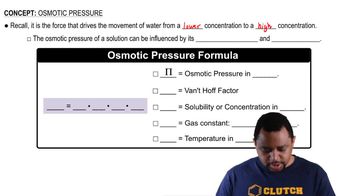


A glucose solution contains 55.8 g of glucose (C6H12O6) in 455 g of water. Determine the freezing point and boiling point of the solution.
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. a. 0.100 m K2S b. 21.5 g of CuCl2 in 4.50⨉102 g water
Calculate the freezing point and boiling point of each aqueous solution, assuming complete dissociation of the solute. c. 5.5% NaNO3 by mass (in water)