Textbook Question
An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 °C. Assume a van't Hoff factor of 1.9 for all ionic solutes. a. KCl
2015
views

 Verified step by step guidance
Verified step by step guidance
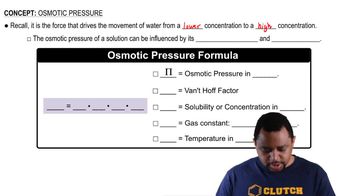
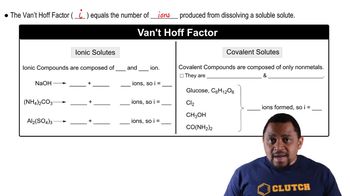

An isotonic solution contains 0.90% NaCl mass to volume. Calculate the percent mass to volume for isotonic solutions containing each solute at 25 °C. Assume a van't Hoff factor of 1.9 for all ionic solutes. a. KCl
A solution of a nonvolatile solute in water has a boiling point of 375.3 K. Calculate the vapor pressure of water above this solution at 338 K. The vapor pressure of pure water at this temperature is 0.2467 atm.
The density of a 0.438 M solution of potassium chromate (K2CrO4) at 298 K is 1.063 g/mL. Calculate the vapor pressure of water above the solution. The vapor pressure of pure water at this temperature is 0.0313 atm. (Assume complete dissociation of the solute.)